Molecular Chaperones and Cancer Research

Our research is focused on the development and integration of computational and experimental approaches for high-throughput ligand screening and design of kinase inhibitors, understanding of the molecular chaperone mechanisms at atomic resolution in order to facilitate discovery of novel anti-cancer therapies.
Computational Studies of the Hsp90 Chaperone and Cancer Research
Our Research is focused on the development and integration of computational and experimental approaches with the goal to advance understanding of the molecular chaperone mechanisms at atomic resolution and facilitate discovery of novel anti-cancer therapies.
Our Projects are :
- Development of novel computationalapproaches for molecular modeling of Hsp90dynamics, ligand-based allosteric modulation and inter-domain communication.
- Integration of computational and experimental approaches into a system biology platform for structure-based characterization of Hsp90 binding mechanisms.
- Computer-based ligand screening, design and biological validation of novel Hsp90 inhibitors
These approaches allowed to develop and validate mathematical and structural models of Hsp90 allosteric regulation and signal communication pathwayS. Client proteins of Hsp90 include protein kinases, transcription factors, and other proteins that serve as nodal points in integrating cellular responses to multiple signals. Deregulation of pathways involving these proteins is commonly associated with cancer pathologies. By disabling multiple signaling circuitries, Hsp90 inhibition provides a novel and powerful therapeutic strategy in cancer research, selective for specific cancer mechanisms, yet broadly applicable to disparate tumors with different genetic signatures. Hsp90 Inhibition can suppresses signaling of kinase cancer mutant clients and overcomes drug resistance. Computational profiling and systems-based approaches are integrated with experimental strategies into a systematic platform for selective targeting of Hsp90-kinase protein networks. The developed allosteric Hsp90 modulators can function as specific and personalized therapeutics for inhibiting protein kinase clients and cancer mutants. We have established a close partnership and are involved in collaborative efforts with a number of prominent Hsp90 research groups including Dr. Matts (Oklahoma State University), Dr. Neckers (National Cancer Institute), Dr. Altieri (University of Massachusetts Medical School), Dr. Agard (UCSF), Dr. Pearl (The Institute of Cancer Research, UK) and others. The expertise of our research team and cross-disciplinary collaborations with the Hsp90 experts in molecular and cell biology, biochemistry, NMR, and structural biology will help to develop and validate computational approaches, refine and enhance experimental tools to advance our understanding of the molecular chaperone mechanisms.The insights about molecular mechanisms and function of molecular chaperone are employed in chemical genomics-based profiling, design and biological validation of novel therapeutics of signal transduction networks.
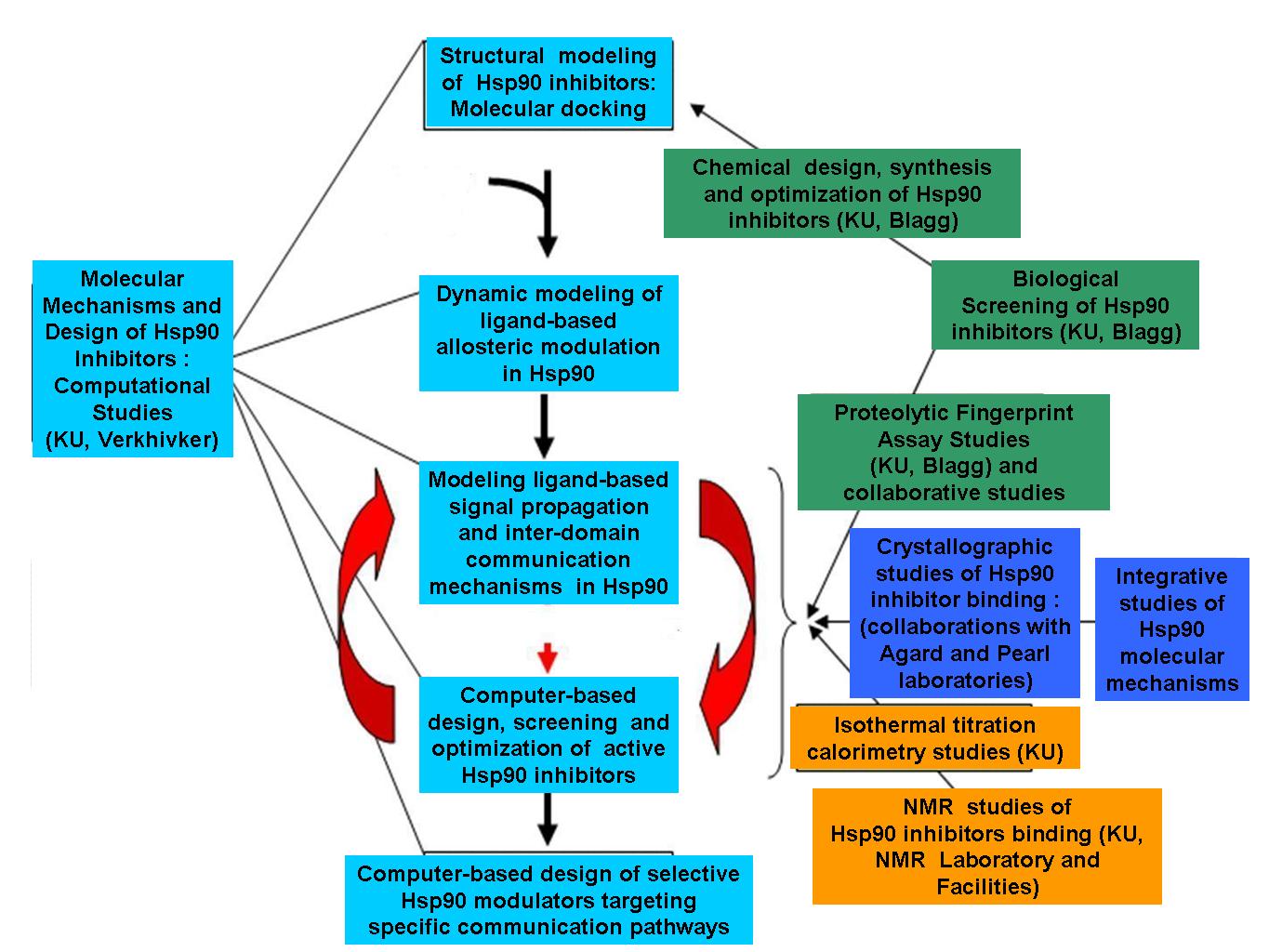
Integrative pipeline of computational and experimental approaches for discovery of allosteric Hsp90 modulators as personalized anti-cancer therapeutics